ISUS Nitrate Sensor
SUMMARY: Since November 2004, a Satlantic ISUS nitrate sensor has been integrated with a Seabird 911+ CTD-Rosette system deployed on CalCOFI cruises. Cruises typically occupy 75 stations, collecting approximately 1400 discrete seawater samples throughout the water column. The discrete seawater samples are analyzed at-sea for nitrate, nitrite, silicate, phosphate and ammonia within 24 hours of collection. The ISUS voltage data are processed along with other sensor data using Seabird’s SBE Data Processing Suite. Processed CTD-ISUS data are merged with bottle data. The ISUS voltages are plotted versus corresponding nitrate data, generating a voltage-to-nitrate regression. These regression coefficients are applied to all ISUS voltages, converting voltages to estimated nitrate.
1. Principle
The Satlantic ISUS (In Situ Ultraviolet Spectrophotometer) is a real-time, chemical-free ultraviolet spectrophotometer detecting absorption characteristics of inorganic compounds in the UV light spectrum. The ISUS uses the UV (200-400 nm) absorption characteristics of nitrate and bromide to provide in situ measurements of their concentrations in solution. The sensor has four key components: a stable UV light source, a UV spectrophotometer, a bifurcated fibre optic sampling probe, and a processing microcomputer housed in a pressure case rated to 1000 meters. The ISUS measures the in situ absorption spectrum and then uses the calibrated coefficients and a least-squares curve fitting routine to calculate an absorption spectrum matching the measured spectrum. It then calculates the concentrations of nitrate and bromide required to generate the matching spectrum. This response is exported to the Seabird CTD as voltage logged with other sensor data at 24Hz.
2. CTD Integration
|
2.1. |
Clean the sensor: prior to mounting, the ISUS sensor optical path is cleaned with an alcohol-dipped cotton swab following the method described in the Satlantic ISUS manual. Basically, the alcohol-dipped swab it pulled across the optical surfaces in one direction. Using a fresh swab each time, the process is repeated until the optical surface is clean. This process should be performed whenever the sensor response seems effected by bio-fouling. |
|
2.2. |
The ISUS is mounted on the rosette so the sensor has unobstructed seawater flow. An ISUS battery is mounted nearby to provide power (ISUS v1 or v2 draws more amps at startup than can be provided by the Seabird 911+ CTD). |
|
2.3. |
Cable connections: connect the ISUS analog-out port to an open CTD channel; rig the battery cable so it can be easily, securely attached to the ISUS power connector several minutes prior to deployment. Note that internal data logging will begin when the battery is attached but the sensor generates better in-situ data when warmed-up for several minutes. |
|
2.4. |
Software setup: Seasave, the Seabird CTD data acquisition software, will record the voltage from the ISUS on the channel it is installed. To display a real-time estimated nitrate cast profile, a ‘user-polynomial’ is setup to display ISUS data. Coefficients from a previous discrete-nitrate vs ISUS voltage comparison are entered as second-order polynomials. |
3. Data Processing
|
3.1. |
Using Seabird’s SBE Data Processing Suite, apply the 911+ recommended (by the help or data processing manual) modules:
3.1.1 Datcnv – ascii-formatted cnv files are generated for all casts 3.1.3 Filter – low pass filter A equal to 3 secs is applied to ISUS voltage; low pass filter B equal to 0.15 secs applied to pressure 3.1.4 AlignCTD – oxygen sensors 4 secs offsets applied 3.1.5 Cell Thermal Mass – standard corrections applied to both conductivity sensors 3.1.6 Derive – depths, salinities, oxygens, densities, potential temperatures, specific volume anomaly, dynamic meters (heights) are (re)calculated using processed cnvs. 3.1.7 Ascii-out - export the basic parameters: scans, pressure, temperatures, salinities, oxygens, depths & voltages to asc files. |
|
3.2. |
A preliminary IEH (legacy data processing & archival ascii format) data file of bottle sample data is generated using CODES & DECODR, two ‘in-house’ data processing programs. |
|
3.3. |
The CTD data is merged with bottle data using another ‘in-house’ developed Windows software program, BtlVsCTD.exe. 3.3.1 During each CTD cast, Seasave generates a .bl file which indexes the scan value when a bottle-trip is initiated and when the bottle closure is confirmed. Using the .bl file indexes as end points, BtlVsCTD bin-averages 4 seconds of CTD data prior to the bottle closures. 3.3.2 The matching bottle data are appended to the comma-delimited CTD data records into a csv. This csv includes data from all CTD records with matching bottle data. 3.3.3 Importing the csv into Excel, the 4-sec average ISUS voltages are plotted vs the bottle nitrate data. A linear regression is applied and the coefficients tabulated. 3.3.5 In addition to ISUS/nitrate, CTD oxygen (ml/L) and fluorometer voltage are regressed vs bottle data, coefficients tabulated; CTD salinities are compared to bottle salts > 340m, offsets derived for both conductivity sensors. 3.3.5 BtlVsCTD.exe - using the bottle vs CTD regression/correction coefficients, csvs of 1m bin-avg upcast CTD data merged with bottle data are generated. These data (temperature, salinity, oxygen, chlorophyll, & nitrate) vs depth are plotted using Matlab for point-checking and CTD data-quality assessment. |
|
3.4. |
Final CTD data processing is performed using Seasoft modules. 3.4.1 CTD data files are split into down and up casts using the Split module. 3.4.2 The Loopedit module is applied to downcast data; Settings: type = ‘Fixed Minimum Velocity’, ‘Minimum CTD Velocity’ = 0.0333m/s, ‘Bad Scans Excluded’ 3.4.3 Binavg module applied to both down and up cast files, averaging CTD data into 1 meter depth bins. 3.4.4 Ascii-out of up and downcast CTD data. |
|
3.5. |
Once final bottle data are available, they are merged with final CTD data using BtlVsCTD.exe. Resulting csvs are plotted using Matlab for final data QC. Data are considered final once the final plots are assessed and final corrections applied, if necessary. |
4. Calculations
4.1 Linear regression of ISUS voltage vs discreet nitrate data generates cruise-average correction coefficients.
4.2 BtlVsCTD calculates individual station regressions of ISUS voltage vs discreet nitrate data. This ‘on-the-fly’ linear regression generates station-specific corrections coefficients which are applied to the specific cast.
Both cruise and station-corrected nitrate estimates (and the coefficients) are tabulated in the final csvs.
5. Equipment/Supplies
· Satlantic ISUS v2 Nitrate Sensor
· Three 12v Wet-labs rechargeable battery packs
· ISUS analog signal to Seabird 9 interface cable
· ISUS power to battery cable
· ISUS Rs-232 interface cable to download internal data files
· Windows laptop with serial interface to program the ISUS and download data.
· Alcohol & cotton swabs
· Nutrient collection tubes for seawater samples
· Seal QuAAtro nutrient analyzer & in-house analyst
6. References
· Johnson, K.S.; & L.J. Coletti. 2002. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep Sea Research I 49: 1291-1305.
· Maillet, Gary and Geoff MacIntyre. 2009 Real-Time Monitoring of Nitrate With the Satlantic-ISUS Sensor. Online at: http://www.meds-sdmm.dfo-mpo.gc.ca/isdm-gdsi/azmp-pmza/documents/docs/bulletin_6_10.pdf
· Satlantic Incorporated. 2005. MBARI-ISUS V2 Operation Manual, Document Number: SAT-DN-272, Revision G.1, August 2006
ISUS NITRATE HISTORY
The original methods description was written Feb 2010 by J. Wilkinson. Changes to the method or instrument are listed below.
Changes
|
Cruise/Ship |
Date |
Author |
Description |
| 1611SR+ | 11-06-2016 | J.Wilkinson | ISUS firmware upgraded to v3 allowing ISUScom software usage & sensor recalibration by SIO-CalCOFI, plus batch downloading via USB cable |
| 1203SH+ | 3-24-2012 | L. Ekern | Discrete nitrate sample analysis now preformed on Seal QuAAtro Analyzer by an in-house technician rather than being contracted out. |
|
0810NM |
10-14-2008 |
J.Wilkinson |
ISUS not deployed this cruise. |
|
0610RR |
10-21-2006 |
J.Wilkinson |
new LTER ISUSv2 deployed for the first time this cruise. |
|
0607NM |
07-01-2006 |
J.Wilkinson |
ISUSv1 (crushed on LTER Process Cruise P200605) not deployed this cruise. |
|
0411RR |
|
|
|
PRIMARY PRODUCTIVITY CHANGES
A description of the method was written February 2010 by D. Wolgast. Changes to the method are listed below.
Radioactive Specific Activity Changes
| Section | Date | Prepared By | Description |
|---|---|---|---|
| 3.3 | 6/10/2016 | D. Wolgast | 25mCi diluted to 32uCi/ml Batch 0509YW4, 800mls |
| 3.3 | 8/8/2014 | D. Wolgast | New 14C Stock: diluted to 69.40µCi/ml; Batch 0500ZV4 (replaces 0509G57 orig. UCSD barcode), used for (1411) November 2014 to present |
| 3.3 | 9/8/2010 | D. Wolgast & D. Faber | New 14C Stock: diluted to 308.37µCi/ml; Source: MP Biomedicals, barcode 0508YD5, used for (1011) September 2010 until July 2014 (1407) |
| 3.3 | 12/15/2008 | D. Wolgast & D. Faber | New 14C Stock: diluted to 284.23 µCi/ml; Source: MP Biomedicals, batch 081215, used for (0901) January 2009 until August 2010 (1008) |
| 3.3 | 3/9/2007 | D. Wolgast & J. Sheldon | New 14C Stock: diluted to 271.32 µCi/ml; Source: MDS Nordion, batch 070308, used for (0704) April 2007 until November 2008 (0810) |
| 3.3 | 6/23/2005 | D. Wolgast | New 14C Stock: diluted to 335.90 µCi/ml; Source: MP Biomedicals, batch 0506, used for (0507) July 2005 until January 2007 (0701) |
| 3.3 | 4/5/2005 | D. Wolgast | New 14C Stock: diluted to 215.76 µCi/ml; Source: MP Biomedicals, used for (0504) April 2005 |
| 3.3 | 10/25/2004 | D. Wolgast | New 14C Stock: diluted to 2351.46µCi/ml; Batch 0411, used for (0411) November 2004 until January 2005 (0501) |
| 3.3 | 3/3/2004 | J. Sheldon | New 14C Stock: diluted to 75.03 µCi/ml; Source: ICN Biomedicals, batch 0402, used for (0403) March 2004 until July 2004 (0407) |
| 3.3 | Mar-03 | D. Wolgast | New 14C Stock: diluted to 41.48 µCi/ml; Source: ICN Biomedicals, batch 0324, used for (0304) April 2003 until January 2004 (0401) |
| 3.3 | Mar-03 | D. Wolgast | New 14C Stock: diluted to 42.38 µCi/ml; Source: ICN Biomedicals, batch 0302, used for (0204) April 2002 until February 2003 (0302) |
| 3.3 | 11/17/1995 | D. Wolgast | New 14C Stock: diluted to 51.45 µCi/ml; Source: ICN Biomedicals,batch 03Z58 used for Nov 1995 until January 2002 (0201) |
Radioactivity added
| Cruise | Radioactivity added µCi | Comments |
|---|---|---|
| 1704 | ||
| 1701 | 5.89 | |
| 1611 | 6.58 | |
| 1607 | 6.63 | New Batch 0509YW4 |
| 1604 | 9.26 | |
| 1601 | 8.64 | |
| 1511 | 10.36 | |
| 1507 | 11.64 | |
| 1504 | 12.18 | |
| 1501 | 8.74; 8.08 | Due to the decreasing activity of the isotope stock through the cruise the prodo samples were processed in two batches. |
| 1411 | 10.21 | |
| 1407 | 7.783 | |
| 1404 | 9.481 | |
| 1402 | 9.47 | |
| 1311 | 10.239 | |
| 1307 | 11.47 | |
| 1304 | 11.03 | |
| 1301 | 11.28 | |
| 1210 | Varied | |
| 1207 | 27.68; 12.95 | |
| 1203 | 12.69 | |
| 1202 | 12.36 | |
| 1110 | 11.76; 10.15 | Due to the decreasing activity of the isotope stock through the cruise the prodo samples were processed in two batches. |
| 1108 | 13.78 | |
| 1104 | 6.992 | |
| 1101 | 49.66 | |
| 1011 | 50.64 | |
| 1008 | 25.6 | |
| 1004 | 40.05 | |
| 1001 | 40.6 | |
| 0911 | 43.19 | |
| 0907 | 43.38 | |
| 0903 | 45.75 | |
| 0901 | 46.59 | |
| 0810 | 40.27 | |
| 0808 | 44.36 | |
| 0804 | 45.8 | |
| 0801 | 52.29 | |
| 0711 | 47.95 | |
| 0707 | 48.55 | |
| 0704 | 53.14 | |
| 0701 | 44 | |
| 0610 | 56.1 | |
| 0607 | 63.1 | |
| 0604 | 59.1 | |
| 0602 | 59.1 | |
| 0511 | 59.1 | |
| 0507 | 64.74 | |
| 0504 | 41.58 | |
| 0501 | Variable due to problems with acid/Teflon and bicarbonate substrate. | |
| 0411 | 32.3-41.66 | Transition to higher activity to facilitate DO14C assay. |
| 0407 | 15 | |
| 0404 | 15 | |
| 0401 | 8.3 | |
| 0310 | 8.3 | |
| 0307 | 8.3 | |
| 0304 | 8.3 | |
| 0302 | 8.48 | |
| 0211 | 8.48 | |
| 0207 | 8.48 | |
| 0204 | 8.48 | Stock diluted and stored in cleaned polycarbonate and Teflon |
| 0201 | 11.3 | Stock in glass sealed ampoules |
| 0110 | 11.3 | Stock in glass sealed ampoules |
| 0107 | 11.3 | Stock in glass sealed ampoules |
| 0104 | 11.3 | Stock in glass sealed ampoules |
| 0101 | 11.3 | Stock in glass sealed ampoules |
| 0010 | 11.3 | Stock in glass sealed ampoules |
| 0007 | 11.3 | Stock in glass sealed ampoules |
| 0004 | 11.3 | Stock in glass sealed ampoules |
| 0001 | 11.3 | Stock in glass sealed ampoules |
| 9910 | 11.3 | Stock in glass sealed ampoules |
| 9908 | 11.3 | Stock in glass sealed ampoules |
| 9904 | 11.3 | Stock in glass sealed ampoules |
| 9901 | 11.3 | Stock in glass sealed ampoules |
Methodological Changes
|
Section |
Date |
Author |
Description |
|---|---|---|---|
|
|
Apr-05 |
D. Wolgast |
The procedure for calculating cruise 14C specific activity for the productivity assay was changed to reflect daily 14C additions averaged over the course of a cruise. the six dark bottles have one milliliter removed, added to ethanolamine spiked scintillation cocktail for counting. Previously specific activity was calculated for a batch of stock and used for the entire cruise. The new method served to check pipeting, inoculation amounts and any changes in volatile 14C stocks. |
|
0411 |
|
D. Wolgast |
Transition to higher activity to facilitate DO14 C assay, 10ml split removed from samples for 14 C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
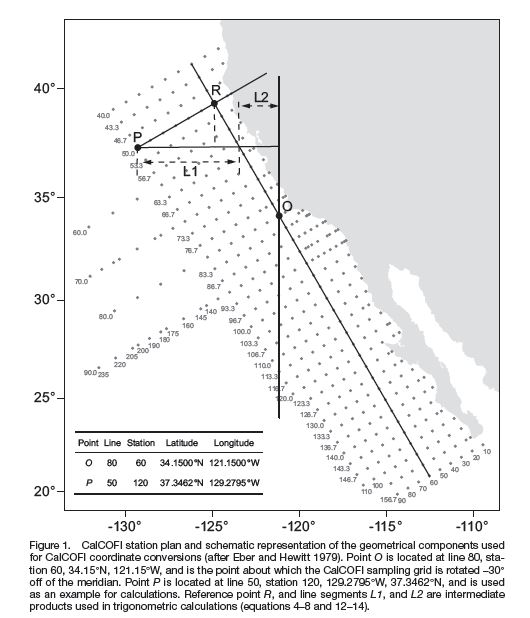
 Matlab scripts by Robert Thombley, SIO-CalCOFI with Sep2014 error correction by Augusto Valencia, UABC; based on Weber & Moor 2013.
Matlab scripts by Robert Thombley, SIO-CalCOFI with Sep2014 error correction by Augusto Valencia, UABC; based on Weber & Moor 2013.
Link to MatLab downloadable code authored by Robert Thombley, SIO-CalCOFI
(Abstract from pdf) "Converting between geographic coordinates in latitude and longitude and the line and station sampling pattern of the California Cooperative Fisheries Investigations (CalCOFI) program is a commonly required task for conducting research on the California Current ecosystem. This note presents several corrections and clarifications to the previously published algorithms for performing these conversions. We include computer code to implement the algorithms in Java™1, Perl, Python, and R. We note that freely available code to conduct the conversions in Fortran, Matlab®2, JavaScript™, and Visual Basic®6 has previously been published, and an online conversion tool is also available. A future version of the PROJ.4 cartographic projections library will also include support for CalCOFI conversions, thereby allowing for convenient conversions using the GRASS GIS, PostGIS, Python, Perl, R, and many other programs and programming languages."
For individual language - Java, Perl, Python, or R - implementations & downloads, see sections below
PRIMARY PRODUCTIVITY
OVERVIEW: Primary production is estimated from 14C uptake using a simulated in situ technique in which the assimilation of dissolved inorganic carbon by phytoplankton yields a measure of the rate of photosynthetic primary production in the euphotic zone.
1. Principle
Seawater samples are incubated with a radioactive substrate to determine the incorporation of inorganic carbon into particulate organic carbon due to photosynthesis at selected light levels. The data have units of mg-carbon per m3 per half day.
2. Productivity Cleaning Procedures
|
2.1. |
Micro-90 Cleaning solution is diluted to 2% solution using de-ionized water (DW). Hydrochloric acid (HCl) Trace Metal Grade, Fisher Scientific, solution (1.2M) diluted with DW. Acid-washing of Teflon should be done with great care as Teflon is porous to HCl which can compromise dilute basic stock solutions of 14C -bicarbonate. |
|
2.2. |
250 ml polycarbonate incubation bottles are filled to capacity with 2% MICRO for 3 days with the cap on in an inverted position. Next, rinse all Micro away and then rinse down the walls with 20 -30mls 10%HCl and recap and shake to acid rinse inside bottle. This should be left overnight 12-16 hours. The acid is removed by rinsing the bottles three times with milliQ clean water before air drying. |
|
2.3. |
10 liter rosette sample bottles are cleaned with a 2% MICRO soak for 3 days, rinsed with de-ionized water and then dipped in 10% metals free HCl. Caps, special coated springs and valve assemblies are also cleaned with a 2% MICRO soak for 3 days and then rinsed with de-ionized water and dried. |
|
2.4. |
All lab ware to be used is cleaned in this manner. |
3. Preparation of Isotope Stock
|
3.1. |
To prevent contamination of self or solutions, work with the isotope stock is performed wearing vinyl gloves. |
|
3.2. |
A solution of 0.3 g of Na2CO3 anhydrous (ALDRICH 20,442-0, 99.995%) per liter Milli-Q filtered DW in a Micro cleaned 1 liter Teflon bottle to yield a concentration of 2.8 mM Na2CO3. This solution is filtered through 0.2µM Nucleapore filter to remove particulate carbonate. |
|
3.3. |
Concentrated stock, 50ml of NaH-14CO3 (~50-57 mCi mmole; MP Biomedicals LLC.) was diluted with 350 ml the 2.8mM Na2CO3 solution in productivity-cleaned 1 liter polycarbonate graduated cylinder. It has become necessary to pH this up with an ultra clean 1N NaOH solution to raise the pH to ~10. |
|
3.4. |
Specific activity can be checked by diluting the above made solution to working concentrations, ie 50-200µl added to 250ml polycarbonate centrifuge bottle and measuring out triplicate 1ml portions into beta ethanolamine spiked (1.5%v/v) Ecolume scintillation cocktail. |
|
3.5. |
To check for 14C-organic carbon contamination another working aliquot of 200µl can be placed into a scintillation vial and acidified with 0.5ml 10% HCl and placed on a shaker overnight. This is done in the hood as it liberates 14C-CO2. The acidified dpm should be <0.0001% of the total dpm of the 14C preparation. |
4. Incubation Systems: situ incubation techniques
|
4.1. |
Incubation apparatus consists of seawater-cooled, temperature monitored incubator tubes wrapped with neutral-density screens which simulate in situ light levels. |
|
|
|
|
4.2. |
Six incubation depths are selected, they represent 56, 30, 10, 3, 1 and ~0.3 % light level. These values are estimated using a wand type PAR meter after cleaning tubes and screens covering them. The near surface light level is reduced to 56% using common plastic screening to prevent a lense effect and subsequent cooking of the surface samples. |
5. Sampling
|
5.1. |
Primary productivity samples are taken each day shortly before local apparent noon (LAN). Light penetration was estimated from the Secchi depth (Using the definition that the 1% light level is three times the Secchi depth). The depths with ambient light intensities corresponding to light levels simulated by near surface and the on-deck incubators were identified and sampled on the rosette up-cast. Extra bottles were tripped in addition to the usual 20 levels sampled in the combined rosette-productivity cast in order to maintain the normal sampling depth resolution. |
|
5.2.
|
Using a dark sleeve to subdue the light, water samples are transferred to the incubation bottles (250 ml polycarbonate bottles) and stored in a dark box until inoculation.
Triplicate samples (two light and one dark control) were drawn from each productivity sample depth. |
6. Isotope Addition and Sample Incubation
|
6.1. |
Samples are inoculated with 50-200 µl of 14C as NaHCO3 stock solution of sodium carbonate (Fitzwater et al., 1982). |
|
6.2. |
Samples are incubated from LAN to civil twilight in a surface seawater-cooled incubators with neutral-density screens which simulate in situ light levels, corresponding to those from which samples were taken (see 4.2). |
|
6.3. |
At civil twilight the incubation is terminated and the time noted. Sea state and safety is the only exception accepted to delay the end time. |
7. Filtration
|
7.1. |
At the end of the incubation, all bottles have subsamples of 10mls removed for DO14C analysis. The LTER DOC filtrate apparatus consists of a plexi-glass filtration manifold to hold up to 18 scintillation vials over which syringe needles with 0.45um equivalent micro-syringe filters can be passed through stoppers with 25 ml syringe bodies serving as filter funnels. The exception to this is dark bottles are only sampled for DO14C on two each high and low chlorophyll stations. |
|
7.2. |
Additionally, from dark bottles a 1ml sample is placed into beta mercapto-ethanol spiked (1.5%v/v) Ecolume scintillation cocktail to determine the specific radioactivity in the samples. These values are used to calculate an average cruise value after removing outliers. |
|
7.3. |
Finally the samples are filtered onto Millipore HA filters and placed in scintillation vials. One half ml of 10% HCl was added to each sample. The samples are then allowed to sit, without a cap, at room temperature for at least 3 hours (after Lean and Burnison, 1979). |
8. 14C Sample Processing
|
8.1. |
After addition of 10mls of Ecolume cocktail, vials are tightly capped and mixed before vials are counted for up to 10 minutes each for 14C on a Beckman 6100LC liquid scintillation counter set to 1.0% counting precision. |
|
8.2. |
Data is captured to a flat file using Beckman data capture software for Windows in ASCII format. This format is then used to integrate productivity depths into the CalCOFI data processing flow. |
9. Calculations
Data is presented as mean mg Carbon assimilated per meter cubed of seawater for one half light day.
mgC/m3 per one half light day = ((Sampledpm – Blankdpm) x W)/R, where
W = 25200 = 12,000 x A x FT x 1.05
12,000= molecular weight of carbon in milligrams
A = carbonate alkalinity (milliequivalents/liter)
FT = Total carbon dioxide content/ carbonate alkalinity
1.05 is the 14C isotope fractionation factor, reflecting preferential use of C12 over C14 by a factor of 5%
R = dpm added to sample (µCi/200µl x 2.2 x 106)
To better understand this equation and variables see Strickland and Parsons (1968).
10. Equipment/Supplies
· 10 liter pri. prod. cleaned sampling bottles
· Secchi disk
· Re-pipet dispensers for delivering 20µl, 200µl, 0.5ml
· Pipets able to measure 1ml and 10ml
· 250 ml polycarbonate centrifuge bottles
· liquid scintillation counting (LSC) vials
· Seawater plumbed incubation rack with neutral density screening.
· Par meter, wand type (Biospherical Instruments)
· 14C sodium bicarbonate stock solution (MP Biomedicals, LLC)
· Millipore Type HA filters (Fisher Scientific)
· vacuum filtration system including separate device for DOC filtrate capture
· Polycarbonate centrifuge bottles
· Teflon laboratory wares
· vortex mixer
· liquid scintillation counter (LS 6000LC Beckman Instruments, Inc.)
11. Reagents
· Milli-Q filtration/anion exchange water purifier
· Micro-90 Cleaning solution, Cole Palmer Instrument Co.
· HCl for trace metal analysis (Fisher Chemical)
· Na2CO3 (99.995%) Aldrich Chemical
· NaH-14CO3 solution (cat #17441H MP Biomedicals, LLC.)
· 2-amino ethanol (ethanolamine) ACS grade
· Aquasol-II (Dupont)
· Ecolume (MP Biomedicals, LLC.)
12. Re-count check
13. References
· Fitzwater, S. E., G. A. Knauer and J. H. Martin, 1982. Metal contamination and its effect on primary production measurements. Limnol. Oceanogr., 27: 544-551.
· Lean, D. R. S. and B. K. Burnison, 1979. An evaluation of errors in the 14C method of primary production measurement. Limnol. Oceanogr., 24: 917-928.
· Steeman-Nielsen, E. (1951). "Measurement of production of organic matter in sea by means of carbon-14". Nature 267: 684–685.
· Strickland, J. D. H. and Parsons, T. R. 1968. A Practical Handbook of Seawater Analysis pp. 267-278.
CalCOFI Time Series data are over 67 years old. New adopted practices, methods, software and hardware are thoroughly tested to maintain dataset continuity as the program & science evolves. Core measurements are maintained and many new measurements added. CTD temperature sensors, for example, provide data at a much higher resolution than a 20 bottle hydrocast equipped with reversing thermometers pre-9308 (Aug 1993).
Although the CTDs casts on CalCOFI started in 1990, CTD-rosette casts did not replace bottles-on-wire hydro casts completely until Aug 1993 (9308). In 2004, LTER joined the CalCOFI cruises, expanding the seawater analyses, adding new measurements.
Changes of standard practices, methods, software, & equipment will be tabulated here, particularly those affecting the hydrographic data or other data products.
| Cruise/Date |
Measurement, |
Changes |
Comments |
|---|---|---|---|
 |
Methods Timeline Started |
This page is 'dynamic' so new items will be added whenever new methods, measurements, equipment are implemented. Older changes will be added as time-permits and they are remembered. |
|
| 1708SR | CCE-LTER ISUS upgraded to fw v3 | ISUS upgraded to firmware v3 | Seabird acquired Satlantic and took several month to get ISUS service online. Once done, they upgraded the CCE-LTER MBARI-ISUS v2 to firmware v3. Allows easy in-house or at-sea calibration. |
| 1708SR | Oxygen Stable Isotope | Seawater samples collected for OSI | 5ml of seawater collected from two depths (10m & 50m) on 18 stations |
| 1701RL 1704SH |
LARS rosette | 24-btl LARS rosette used | Even though RV Reuben Lasker & FSV Bell M Shimada do not have a LARS CTD deployment system, the height clearance allows the use of the LARS rosette. SIO-CalCOFI will use this rosette as primary unless the height clearance becomes a problem, such as on RV Ocean Starr. |
| 1611SR | First RV Sally Ride | SIO RV Sally Ride used for CalCOFI | RV Sally Ride used for the first time to do CalCOFI station work; acoustics operational but not calibrated |
| 1611SR | RINKO III Optode | RINKO III Oxygen sensor deployed | Optode used on non-basin stations deeper than 500m, where altimeter was not needed |
| 1611SR | Seawater samples | Domoic Acid & Th-234 | Collected ancillary seawater & deploy insitu pumping system |
| pre-1611SR | ISUS (Frank's) Repaired | Peter Frank's ISUS repaired & upgraded to firmware v3 | ISUS was not working so it was sent to Satlantic who repaired it and upgraded the firmware to v3 |
| 1609SR | New research vessel | SIO RV Sally Ride test for CalCOFI | Shakedown cruise for RV Sally Ride to do CalCOFI - new LARS, epoxy-coated rosette deployed |
| 1609SR | LARS epoxy-coated rosette | First use of new 24-bottle rosette | 24-btl white epoxy coated aluminum rosette deployed with stainless steel LARS support |
| 1607OS | Seawater Samples | Domoic Acid samples collected | Ancillary seawater sample collection |
| 1604SH | Nutrient Analysis | New (returning) in-house nutrient chemist | Nutrients run by SIO-CalCOFI analyst DGS |
| 1507OC | Underway Measurements | RV Oceanus MET system logs data into individual data files; combined data are unavailable unless merged manually | Unlike other UNOLS vessels, particularly SIO RV New Horizon, the underway TSG & meterological data are logged at 1Hz into individual files. A combinded data file will have to be generated post-cruise by merging individual sensor data files using the common UTC timestamp. Software pending... |
| 1504NH | Underway Measurements | PCO2 and pH measurements added to CCE-LTER suite |
|
| 1504NH | Nutrient Analysis | Standard matrix; sample vials |
Standards now prepared in low nutrient seawater (collected from the end of lines 93.3 or 90.0 and processed with UV light, filtration and aged before use). New 30 mL polypropylene tubes in use. |
| 06 Aug 2014 | calcofi.com | calcofi.com 2014 online |
Developmental site web.calcofi.com replaces Joomla 1.5 calcofi.com; Joomla 3+ fully implemented. calcofi.com old site moved to old.calcofi.com |
| 1402SH | Nutrient Analysis | New in-house Nutrient Technician |
Nutrients run by SIO-CalCOFI analyst LJE. |
| 1402SH | NCOG DNA/RNA | NCOG sample collection started | NCOG DNA/RNA samples collected at ~4 depths (10m, chl max, 170m, 515m) |
| 1311NH | DIC measurments | Sampling expands |
Dissovled inorganic carbon samples are now drawn at more locations along the cruise track with 14 profile and 8 additional surface water stations per cruise. |
| 1211NH | Rinko Oxygen Optode | RINKO III Optode deployed | RINKO III Oxygen Optode sensor replaced 2nd SBE43 oxygen sensors on stations where the altimeter was not needed - non-basin stations deeper that 500m. |
| 01Mar2013 | web.calcofi.com website update | Upgrading calcofi.com to latest Joomla |
web.calcofi.com started to migrate calcofi.com 1.5 to 3.0; to improve responsiveness with new dynamic templates - desktop, tablet, and smartphone auto-formatting. Security improvements, auto-updating, jQuery & other new features of version 3.+ |
| 1207OS | Nutrient Analysis | New in-house Nutrient Technician |
Nutrients run by former ODF chemist, now a SIO-CalCOFI analyst MTM. |
| 01May2012 | dev.calcofi.com blog started | SIO-CalCOFI Technical Group developmental blog started. |
For metadata: documenting changes in software, data-processing methods, formats, products, & practices. |
| 1203SH | Nutrient Analysis | Instrument and analyst change, new Seal QuAAtro Analyzer purchased. |
Transitioned to in-house nutrient analysis on new Seal QuAAtro Nutrient Analyzer, nutrients run by SIO-CalCOFI analyst DNF. Replacing sample analysis by ODF chemist on an AA3 analyzer. Standards now prepared in artificial seawater. NO3 data calculated using regression coefficients from ISUS voltage vs NO3 for 1202NH & 1203SH look nearly identical. |
| 1203SH | atsea.calcofi.com blog online | Setup to keep notes during quarterly cruises, particularly those related to data, equipment, & generally noteworthy. |
Connected-linked to CalCOFI's Twitter feed |
| 1202NH | Nutrient Analysis | Last cruise where nutrients were run by ODF chemist on an AA3 analyzer. |
|
| 1202NH+ | Data Processing | IEH retired as primary data processing file format. Sta.csvs and casts.csv adopted. | After parallel-processing (IEH & csv file format) 1104, 1108, 1110 cruise data. Sta.csvs & casts.csv data processing is being used to process, merge, quality-control, publish all hydrographic data. IEH-format is a data product along with the hydrographic database. |
| 1108 | All measurements & data products | Hydrographic data are processed using both old & new methods and compared. |
The cruise is once again processed twice, in parallel, using the IEH old-school method and the new csv-format method. GTool development & refinement continues as sta.csvs & casts.csv formats are improved. Final data products are compared for agreement. This is the first cruise to generate data products using new csv-database data processing methods not based on IEHs. |
| 1104 | GTool Matlab program developed | SIO-CalCOFI Technical Team implement a new graphical matlab tool to point-check CalCOFI hydrographic data |
MGS, in cooperation with the CCTG, develops GTool to replace Andyplots, our legacy method to visually inspect all bottle data on one figure. CTD continuous data are plotted with bottle parameters to cross-check and eliminate fliers. |
| 1104 | All measurements; data products | Hydrographic data are processed using both old & new methods and compared. |
The cruise is processed twice, in parallel, using the IEH old-school method and the new csv-format method. GTool, a matlab plotting & data-quality control program script, is developed to ingest CTD & sta.csvs to graphically assess CTD & bottle data-quality. Final data products are compared for agreement. This should be the last cruise processed using 00/20/22 & IEHs. |
| 1101 | Reported standard level O2 | CTD primary or secondary oxygen sensor bottle--corrected measurements are reported instead of interpolated standard level (ISL) oxygen values | Pretains to final, standard-level bottle data reported in all data products - with the reliability of the SBE 43 oxygen sensors and consistently high data quality when calibrated bi-yearly (twice/year) and bottle-corrected. Bottle-corrected CTD primary or secondary oxygen values (whichever sensor is performing better that cast) are reported instead of interpolated (calculated) standard level bottle oxygen values. |
| 1101 | Data storage format, ieh phase out begins; all measurements affected | Sta.csv & casts.csv implementation begins, replacing 00/20/22 & IEH data process methodology. IEH-method is used as CSV-method is developed. |
Bottle data & CTD data processing up til now have been relatively separate although data-quality cross-check are common. Other than CTD temperatures, all hydrographic data reported in IEHs or hydrographic database are bottle sample measurements. In 2011, our new data processing strategy merges the bottle & CTD starting at seawater sample collection. CESL generates individual sta.csvs combining CTD & bottle data immediately after seawater sample collection. Casts.csv is also generated and contains station specific information, replacing the Station Cast Description & Weather files (stacst & weather). |
| 1101 | DECODR | DECODR migration to the new sta.csv and casts.csv data-processing scheme in full development |
DECODR (Data Entry & Compile Oceanographic Data Reports) program modules are adapted to process either old or new hydrographic data formats. Sta.csvs & casts.csv adaptations are implemented and different data products are generated directly or by generating an IEH first then data products. |
| 0901 | DIC measurements resume | DIC (dissolve inorganic carbon) sampling resume after a hiatus. |
DIC, aka Keeling, samples were commonly taken on two CalCOFI stations but the new sampling scheme covers several additional stations and depths. |
| 2008 | Nutrient Analysis | Began ammonium analysis |
Nutrient analysis expanded to include ammonium. |
| 0701 | Salinity measurement | SalReCap - Salinity Record & Capture program introduced, replacing the PSal (DOS shell executable). |
SalReCap, Windows program developed by SIO-CalCOFI, replaces ODF-developed PSal, a DOS executable. Immediate comparisons to CTD salinities during analysis becomes available. |
| Apr-05 | Primary Productivity | New specific activity procedure | The procedure for calculating cruise 14C specific activity for the productivity assay was changed to reflect daily 14C additions averaged over the course of a cruise. The six dark bottles have one milliliter removed, added to ethanolamine spiked scintillation cocktail for counting. Previously specific activity was calculated for a batch of stock and used for the entire cruise. The new method served to check pipeting, inoculation amounts and any changes in volatile 14C stocks. |
| 0411 | Ancillary measurements such as HPLC, DOM, Size fractionations, Epifluorescence | LTER affiliation on CalCOFI cruises begins |
CCE-LTER (California Current Ecosystem Long Term Ecological Research Site) is established and LTER participation on all CalCOFIs begins. A large suite of additional measurements, particularly seawater samples from the rosette, and PRPOOS vertical plankton tow are added to CalCOFI hydrography. |
| 0310 | Oxygen measurement | Autotitrator replaces manual modified Winkler titrations |
After substantial assay-comparative testing, an ODF-developed oxygen auto-titrator is used to titrate discrete, rosette-bottle, seawater oxygen samples at-sea. New oxygen data format & data processing module is introduced in DECODR. |
| 0204 | CalCOFI Data Report | Last bound hardcopy of the bi-annual CalCOFI Hydrographic Data Report is published, CC Reference 03-01 31 July 2003 |
CalCOFI Hydrographic Data Reports will continue being published electronically as pdfs for global distribution. Bound hardcopies, sent my mail, to different libraries & institutions will stop. |
| 0104 | Chlorophyll measurement | FLog, chlorophyll data logging fluorometer program introduced |
Prior to FLog, our 24+hr acetone cold-extracted chlorophyll samples were manually logged by hand to a sample form then key-entered into CODES. FLog records fluorometer values automatically in a data-processing friendly format so no transcription is required. |
| Jun 2001 | Seasave for Windows | Seabird releases Windows versions | Seasoft/Seasave & SBE Data Processing software for Windows 98/NT released by Seabird |
| 2000 | CTD Fluorometer | Seapoint Fluorometer |
Seapoint Chlorophyll Fluorometer in use. |
| 9807 (Jul 1998) | CTD Seasave | Con file readable by Seasave v5 | con files prior to 9807 will not open it Windows SBE Data Processing software v5 or v7 |
| 9308 | Temperature measurement | 4sec ave (prior to bottle closure) CTD temperatures replace reversing thermometer measurements |
CTD temperatures are the only measurement entering the hydrographic timeseries (until 2011 when CTD standard level temperature, salinity, & oxygen replace interpolated values). |
| 9308 | Seabird CTD-Rosette w/ 24-10L Niskin-type PVC Bottles | Replace 20 bottle hydro cast & 6 bottle prodo cast |
10L Niskin-like bottle constructed by Research support have plastic-coated springs, nylon lanyards, Viton or nitrile o-rings. No metal, rubber, or latex come incontact with seawater samples. Bottles are disassembeld & 'productivity-cleaned' between cruises. |
| 1993 | CTD Fluorometer | SeaTech Fluorometer |
1993-2000 |
| 1973 | Chlorophyll measurement | Began |
Discrete chlorophyll analysis was added to the hydrographic dataset. |
| 1961 | Nutrient Analysis | Expands beyond phosphate |
Nutrient analysis expanded to include silicate, nitrate and nitrite. |
|
|
CALCOFI PRIMARY PRODUCTIVITY PROTOCOL
(H:\word\instruct\ANALYTICAL\Prd_inst_2012SH.doc 2/2012)
PRE-CRUISE PREPARATIONS
1. Check well in advance of cruise to be sure there is enough isotope ready to be used. This amount may vary depending on specific activity desired. Also, check scintillation fluid and all other supplies on the supply list.
2. Be sure a copy of this protocol and a “Request for Isotope usage on SIO Vessels” (see sample forms section at the end of this Protocol) have been sent to the ship scheduling office. These items should be submitted three months in advance if possible. Submit these also for cruises NOAA vessel just as though it were an SIO vessel to extend licensing to the vessel.
3. Incubator tubes should be cleaned and calibrated prior to each cruise. After the Incubator tubes are calibrated, a new “desired depth” sheet must be prepared using the new calibration values. The new calibration values must also be entered into the PIC module of CODES for determining sampling depths at sea.
4. Polycarbonate bottles used for incubations should be cleaned with MICRO and acid rinsed prior to each cruise.
5. Rosette bottles used for Productivity sample collection must be MICRO cleaned and acid rinsed prior to each cruise.
6. Make a set of stick on labels scintiallation vials with sequential numbers for use on the cruise. Each CalCOFI productivity experiment uses 18 vials; typical cruises have 15 or 16 experiments.
ON SHIP PREPARATIONS
7. While setting up aboard ship make sure the Van drain hose is tightly connected. Check to be sure it does not leak and has unimpeded flow. The end of this hose should extend over the side and slightly below the sea surface. Make sure there is no chance of leakage from this line onto the ship deck. This hose should be clearly labeled as Van waste, and reserved for use only as a Van drain.
8. Check to be sure the drain valves beneath the sink are set initially so the sink will drain overboard. Be sure you understand how the valves are to be set for overboard draining of the sink.
9. Check to be sure there are seven liquid waste jug/vacuum traps empty and ready for use. One should be mounted at the left side of the radioactive filtration basin and connected to the vacuum pump system, ready to go for filtrations. Six should be in the waste containment tray on the floor below the counter.
DAILY AT SEA OPERATIONS
1. Early in the day (or late the previous day) figure out which station will be the productivity station and calculate Local Apparent Noon (LAN).
2. Prior to the CTD cast determine the Secchi depth. During the CTD down cast use the PIC module of the Codes program to integrate the productivity depths (or if necessary “Productivity Cast Worksheet” as set by the Secchi depth, into the hydrographic cast depths, as set by the hydrographic parameters observed on the down cast. If necessary add extra depths to the cast to preserve close spacing where needed for the hydrographic parameters. Be conservative, it is better to over sample than to leave gaps. Begin filling out the “Primary Productivity Data” sheet.
3. Ideally the CTD cast should be timed so the samples can be drawn, inoculated almost immediately, and placed in the incubators at LAN. Ideal timing is seldom possible. The CTD cast may be done early, with the CTD coming back on deck as much as 1.5 hours prior to LAN. If the cast is done early, the samples should be set aside in a cool dark place until they are inoculated. Make every effort possible to have the samples in the incubator by LAN +/- 15 minutes. In case of disasters and delays, samples may go into the incubator as late as LAN + 1 hour. If delays beyond LAN + 1 hour are encountered the experiment may be cancelled.
4. Productivity samples are drawn into the numbered set of 280 ml Polycarbonate bottles, two clear bottles and one dark bottle at each productivity sample depth. Productivity samples are normally drawn after all the other samples. If time is running short, they may be drawn immediately following the oxygen samples. While drawing samples, use the dark bottle sleeves and try as much as possible to shield the samples from light.
5. Samples are inoculated with 0.2 ml of C14 stock (approximately 5-20 micro Curies, depending on the precise concentration of the stock) using a repeating pipette fitted with a small diameter extension tip. The first squirt from the Eppendorf pipette is not accurate, so it must be squirted back into the ampoule. Inoculations must be carried out in subdued light, using the “night lights” and the hood light in the Van. Prior to the inoculation procedure a drain plug will be placed in sink in the event of a spill to prevent isotope being carried downstream into the ocean to allow time for isotope to be treated with acid cleaning solution. All inoculations must be carried out in the containment basin area of the Van. Gloves must be worn for this operation. Absorbent paper need not be placed in the containment basin while adding the isotope because the basin is designed so it can be flushed directly into the sink. The leftover isotope must be flushed into the liquid waste jug/vacuum trap to be disposed of later along with the filtrates from the experiment. Pipette tips must be rinsed at least three times, with the rinses drained into the liquid waste jug/vacuum trap. Use one of the 1st filter funnel as a miniature sink for this rinsing. The pipette tips and ampoule parts should then be treated as radioactive trash, and should be put in the plastic "sharps" container provided. The inoculation must be totally finished and the incubation bottles tightly capped before removing the sample box from the containment basin. Do not open the sample bottles for any reason outside of the Isotope Van.
6. Set up the filtration area prior to end of incubation time. Load the filtration funnels with new 25mm Millipore type HA filters. Be sure to note the space left in the waste jug/vacuum trap. We need to fill these jugs ¾ fill line, avoid sucking filtrate into the secondary trap. Three days per jug, this requires careful attention on the part of the technician.
7. Samples should be removed from the incubator at Civil Twilight (CT) +/- 15 minutes. The samples should then be filtered immediately, using 0.45 micron type HA Millipore filters. Filtrations should be carried out under subdued light. Gloves must be worn while filtering and during cleanup following filtration. Do not open the sample bottles until they are over the containment basin. Pour samples into respective filter funnels, ~140 mls at a time. A 1ml subsample is required from the dark bottle to determine the exact activity added to each bottle. To this an equivalent 200ul of ethanolamine has been added to the Ecoscint to keep the 14C in solution. Assign a number series and note the appropriate number in the comments section.
8. Give both the sample bottles and the filter funnels two small rinses (about 20ml) with seawater drawn from a deep Niskin bottle to rinse down any phytoplankton and isotope clinging to the sides. Collect these rinses on the filter and in the liquid waste jug/vacuum trap. While the samples are filtering rinse out the empty productivity sample box with 10% HCl. The sample box rinse can go down the sink. After these rinses put the sample bottles in the sink for further rinsing.
9. Sample filters should then be removed and placed in a LSC vial. Add 0.5 ml of 10% HCl to each filter. Be sure the filter lies flat in the vial and is covered with acid. The vials are then allowed to sit uncapped overnight inside the fume hood. After the filters are removed, put the filter funnels in the sink for further rinsing.
10. Be sure the “Primary Productivity Data” sheet has been completely filled out.
11. Rinse all the Polycarbonate sample bottles, caps, and filter funnels at least three times with large volumes of seawater. No matter what, do not rinse with ships fresh water due to metals contamination. Make all items ready for the next days work. These rinses can be allowed to drain directly over the side. Finally, give each bottle a 10% HCL rinse. At the end of cruise, rinse HCl out of the bottles to prevent it from being put in the waste jug with the Micro rinse in Sverdrup hall.
12. The radioactive filtrate and rinse water in the waste jug/vacuum trap must be retained and returned to EH&S at the end of the cruise. Fill out the “Waste Collection Log” on a daily basis. Label waste accordingly. For logging purposes the "Liquid waste collected" at sea is estimated to be the total activity contained by the vial, minus 0.1 percent estimated to be taken up by the phytoplankton on the filters.
13. Next morning add 10 ml of Liquid Scintillation Cocktail (currently MP Biomedical Ecolume) to each vial and cap the vials firmly and shake them vigorously.
WEEKLY WIPES
1. Wipe test the van. Floor, bench and sink is sufficient for weekly tests. Diagram the wipes on a “Radioisotope Survey and Monitoring Form”. Date the wipes accordingly. Required by Ships Operation/Radiation Safety.
END OF CRUISE
1. Rinse incubator tubes and manifolds with fresh water. Allow the tubes to air-dry and repack them in their bags. Disconnect and pack plumbing and manifolds for storage ashore.
2. While still at sea, thoroughly clean the Van. Wash the bench tops and floor, with a final wipe using dilute HCl. Rinse the filtration manifold with fresh water. Do these cleaning operations before the wipe tests.
3. Wipe test the van. Diagram the wipes on a “Radioisotope Survey and Monitoring Form”.
Complete the “CalCOFI Isotope Balance Sheet” for the cruise and the “Shipboard Radioisotope Usage Form”. The “Shipboard Radioisotope Usage Form” and “Radioisotope Survey and Monitoring Form” must be signed by the P.I. and Chief Scientist. The "usage" form and the "survey" form must be forwarded to the Ship Scheduling office, with copies to E.H. & S. Be sure also to keep copies of all these completed forms in the CalCOFI isotope record notebook.
4. Before the Van is removed from the ship, be sure the drain hose is removed and properly stowed, and that the drain is covered to prevent it from dripping.
HANDLING WASTE
1. Keep a careful watch on the level of filtrate in the liquid waste jug/vacuum trap. When it is filled to the full line, a new jug must be put in place. On a normal CalCOFI cruise it is convenient to switch the liquid waste jug/vacuum trap after three stations, rather than filling it to the maximum. A typical cruise will have about 15 productivity stations, using 5 jugs. Put the new jug in the filtration containment basin, and carefully move the trap top from the old jug to the new jug, taking care not to dribble fluid around. Cap the jug tightly with the cap from the new jug. Immediately fill out and attach a radioactive waste tag. Carefully lift the filled jug from the filtration containment basin and place it in the containment tray on the floor beneath the counter.
2. Prior to the cruise seven vacuum trap/waste, jugs should be prepared.
3. Do not remove the liquid waste jug/vacuum traps from the isotope van while the van is on the ship. Immediately following the cruise, contact EH&S to arrange for pick-up of the waste jugs. They will come to MARFAC to pick them up directly from the van.
GENERAL PRECAUTIONS
1. On board UNOLS vessels involved in natural abundance isotope work shoe covers must be worn, or a pair of shoes may be left in the Van to be worn only while working in the Van.
2. Items from inside the van such as mops, sponges, brooms, chairs, etc., must not be moved from the Van for use in other parts of the ship.
3. If a spill should occur, the liquids sponged up from the floor should be put into a liquid waste jug/vacuum trap using a large funnel. All mop-squeezings from any mid-cruise or end-of-cruise cleaning of the Van floor should also be put into a liquid waste jug/vacuum trap.
4. The fan mounted in the Van is designed to ventilate fumes out. Both acetone vapors and C14 labeled CO2 are vented by the fan. It should be left on throughout the cruise.
5. All handling of open isotope containers should be done over the containment basin in the Van. This should be clear from the protocol above. There is no reason to have an open isotope container anywhere other than over the containment basin at any time during our procedures.
6. The Van must be clearly labeled for Isotope isolation. Be sure all scientific personnel are aware that access to the Van is restricted to authorized personnel only.
NCOG Sampling For DNA and RNA Samples for NCOG Project
Sample all Prodo (~ 16 stations) and Cardinal Stations (10 stations)
Cardinal Stations = Line 90 (120.0, 90.0, 70.0, 53.0, 37.0); 82.47 (Santa Barbara Basin); Line 80 (55.0, 70.0, 80.0, 100.0)
Prodo and Cardinal Stations may overlap: Prodo stations = consistent time; Cardinal Stations = consistent stations
Sample Depths (typically 4 depths)
- Sample 10m (DNA & RNA sample) RNA filter 1 to 8 L mixed layer/surface
- Chl max depth (DNA & RNA sample) RNA filter 1 to 8 L
- 170 m (RNA sample only) RNA filter 6 to 8 L DIC are typically sampled here
- 515 m (RNA sample only) RNA filter 6 to 8 L DIC are typically sampled here
- 3500 m (RNA sample only) RNA filter 8 to 10 L *would really like this sample if available
Shallow Station Depth Sampling
- Bottom Bottle Depth Less than 515m - sample 10m, Chl max depth and 170m (3 depths)
- Bottom Bottle Depth Less than 170m - sample 10m, Chl max depth (2 depths)
- Bottom Bottle Depth Less than 170m and the Chl max is around 10m - sample 10m (1 depth)
Water Budget
If bottles available trip a duplicate bottle at 10m and Chl max depth
If LTER is getting a duplicate 10m, there may need to be 3 bottles tripped at this depth. (First bottle for CalCOFI, second bottle for LTER, and third bottle for NCOG RNA)
If 2 DIC samples need to be drawn from one of the NCOG depth, you may need an additional bottle tripped to have enough water for the NCOG sample.
DNA Sampling (10m and Chl max only)
*LTER and volunteers will take care of the labeling and filtering
Sampled exactly like the POM samples using the same volumes (0.5, 1.04 or 2.2 Liter Bottle)
Volume depends on the amount of Chlorophyll in the water
*May filter smaller volume if water budget is tight or may not filter at all
*Sample from whatever bottle has water available from that depth
Brown sample bottles – Label D(nisken bottle number) eg D18 or D23
Use combusted GF/F, combusted foil and place the two samples into one cryovial
Label foil D(niskin bottle number)
Label Cryovial CC(Cruise) D(cast number), Station, Bottles and Volumes
Example CC1511OC D001
93.26.7
B 6, 9 .5,1.04L
Store in Liquid Nitrogen in the POM section and label Cryocanes D1, D2, ect…
RNA sampling (4 depths)
*Always keep the filter and samples in the dark, do not leave sample unfiltered for more time than absolutely necessary and record any time sample left unfiltered greater than 30 min. on log sheet.
*Make sure sample comes from the extra bottle
**Change out all 4 of the masterflex tubing in the pump head before cruise and approximately halfway through the cruise
-For high Chlorophyll depths (shallow/chl max) may need only ½ full bottle (less than 4 L) but if water is available draw 8 L and filter until desired color change on filter.
-For low Chlorophyll depth (170, 515, 3500m) try to get as much sample as available, ideally 8 L
Label color coded sample bottle - write the nisken bottle number on the color tape
Collect water, set up and label sterivex filters: Cruise, Station, Cast and Bottle
Place sterivex filter back into plastic if filters are labeled early
Example: CC1511OC
93.26.7
C 1 B 6
Organize samples and filtrate bottles
Rotate and secure tubing in pump head and close latches (allows for even wear on tubing)
Place one end of the tubing into the sample and the opposite end with the filter into the filtrate bottles
Start pump, make sure the pump is flowing in the correct direction and check for leak in the tubing or the sterivex filter
Record time in PST for start and end of filtration
*Stop filtering after 30 minutes or when some color is visible on the filter
Pump speed - 330 rpms
Detach sterivex filter from tubing and remove excess water from the filter
- use a 10 ml syringe 3 to 4 times to push any excess water
- flick the filter a few times to get out any excess water
- plug both ends with putty
Place the 10m and Chl max together in on foil pack and the 170m and 515m together in another foil pack
Label Foil: Cruise, Station, Cast and Bottle Numbers
Example: CC1511OC
93.26.7
C 1 B 6 & 9
Place samples in Liquid Nitrogen. Use center cryo-cane for flash freezing samples and then move samples to proper location in the dewar. Best to make foil pack a little large so when you place in cryo-cane the sample does not float out
Measure filtrate with a graduated cylinder and record volume of filtrate for each depth
Cleanup
Pump about 300ml of milli-Q water through the tubing, shake out any excess and rinse the outside of the tubing with the rest of the water in the bottle, wrap tubing up in a Ziploc bag to keep clean throughout the cruise.
Turn pump off and wipe off any excess salt water on pump head
Open latches and allow slack in tubing located in the pump head (tubing will last longer)
Post cruise
Scan data sheets and email to all points of contact; samples will remain in LTER dewar and LTER will coordinate pickup
Historically, when designing the original CalCOFI Line.Stations pattern, the transect lines were plotted perpendicular to the coastline with .0 decimal accuracy. Since reporting lines and stations with decimal accuracy was unnessary at the time, line and station numbers were rounded to whole numbers to save data column space. Computer cards had a limited number of character columns such as 128 columns in 1983 when the IEH ascii format was adopted. Data storage was also limited.
With the addition of 9 SCCOOS stations in 2004, it became necessary to report the Line & Sta numbers with .0 decimal accuracy to resolve stations. For example, to resolve sta 93.3 26.7 from SCCOOS sta 93.4 26.4, we have to report the decimal line.sta numbers in all the data. Since SCCOOS station data have been integrated into the CalCOFI time series, decimal line & station numbering was applied to the entire time series, even before 2004. Lines & stations of older data on ERDDAP or other biological datasets may not be reported to .0.
Line Number Reporting (Pre-2004 = Current): 93 = 93.3, 90 = 90.0, 87 = 86.7, 83 = 83.3, 80 = 80.0, 77 = 76.7, 73 = 73.3, 70 = 70.0, 67 = 66.7, 63 = 63.3, 60 = 60.0
And between line stations like Santa Barbara Basin: 82.47 = 81.8 46.9. So station designation has changed from 90.120 to 90.0 120.0, 93.27 to 93.3 26.7. It should be noted that the Line.sta calculator & algorithm will derive decimal, not integer, line & station numbers from latitude and longitude.
Differences in CalCOFI Line.Sta numbering in the CalCOFI time-series have been discussed especially when relating historical net tow data to hydrographic data.
When comparing time-series datasets, there are times when there are no matches between biological (zooplankton, ichthyoplankton) and physical (temperature, salinity, oxygen) data. Some data may not have matches, but other data may not match because the criteria used miss data, such as CalCOFI Line and Station numbers reported as integer vs decimal..
Here are some CalCOFI practices that may cause data to not match:
- early (1950's) CalCOFI net tows were often done between stations ie nets were tow as the boat transited between stations. So using specific latitude-longitude or CalCOFI Line.Sta as criteria may not match data.
- there are often net tows but no hydro (bottle or CTD) cast & vice versa. On some early cruises, only one 10m bottle was collected on station. On cruises such as in 1979, net tows were done but no bottle casts. NOAA fisheries performs other surveys in the CalCOFI area where net tows are performed, samples collected, & data added to their dataset without matching physical data. CalCOFI cruise reference: http://calcofi.com/field-work/station-info/20-survey-coverage.html
- If the ocean conditions are bad, station operations may only include a bottle or CTD cast and no net tows or vice-versa.
- During the 70's CalCOFI cruises were every three years so there are gaps in the time-series.
Finding criteria to match data should be easier from 1983 to present since cruise & data practices were improved.
To improve matching biological to physical data, especially when including data earlier than 1983, it is recommended to use criteria ranges instead of exact numbers when possible. Date-time windows of 2-4 hours or lat-lon ranges that are within ~2nm of each other. Two nautical miles is still CalCOFI's "on-station" criteria.
One option that may improve data matches in older data is to use Rpt_Line & Rpt_Sta in the hydrographic dataset for CalCOFI Line & Station since these may be rounded.
Another option is to exclude SCCOOS station data when querying rounded (to integer) CalCOFI Line & Station data.
Nutrient Analysis, Nitrate, Nitrite, Silicate, Phosphate and Ammonium
SUMMARY: The phytoplankton macro nutrients nitrate, nitrite, silicate, and phosphate in seawater are analyzed using colorimetric assays. Ammonium concentrations are determined using a fluorometric assay.
1. Principle
Nutrient analysis is performed on a QuAAtro continuous segmented flow autoanalyzer (SEAL Analytical). A sample of seawater enters a reagent stream within a manifold on the analyzer where it undergoes a series of reactions that ultimately produce a colored compound. These compounds absorb light at a specific wavelength. A monochromatic beam of light is passed through the sample and the absorbance is measured. The machine is calibrated with a series of known standards and a standard curve is produced. The intensity of the color produced by the unknown sample is proportional to the concentration of the analyte present. The product of the ammonia method is a fluorescent species; however the same basic principle applies, where the intensity of the fluorescence is directly related to concentration. The methods for silicate and total oxidized nitrogen (TON) are modified versions of those described by used Armstrong et al. (1967) and Gordon et al. (1992). The phosphate determination employs a modification of the method described by Murphy and Riley (1962), and ammonia is analyzed based on the Kerouel and Aminot (1997) fluorometric method.
2. Method Description
Silicate (SiO2)
Silicate is analyzed using a modified technique of Armstrong (1967). An acidic solution of ammonium molybdate is added to a seawater sample to produce silicomolybdic acid which is then reduced to a blue silicomolybdous acid following the addition of ascorbic acid. The amount of blue color produced is proportional to the amount of dissolved reactive silicate in the sample. Oxalic acid is added to inhibit PO4 color interference. The sample is passed through a 10mm flow cell and the absorbance is measured at 820nm.
Nitrate and Nitrite (NO3 and NO2)
A modification of the Armstrong (1967) procedure is used for the analysis of nitrate plus nitrite, or total oxidized nitrogen (TON). For this analysis, the seawater sample is passed through a cadmium reduction coil where nitrate is reduced to nitrite. The efficiency of this reduction is determined by running two equimolar solutions, one containing only nitrate and one containing only nitrite, through the coil. The percent of nitrate converted to nitrite yields the coil efficiency, a factor used to calculate the nitrate concentration. Sulfanilamide is introduced to the sample stream followed by N-(1-naphthyl) ethylenediamine dihydrochloride which complexes with nitrite to form a red azo dye. The stream is then passed through a 10 mm flowcell and the absorbance measured at 520nm.
The same method is employed for nitrite analysis, except the cadmium column is not present. Consequently, only nitrite already present in the sample is measured.
The nitrate concentration is calculated on a “virtual channel” by the following equation, using the coil efficiency, TON, and NO2:
NO3=Nitrate (in sample)
NO2= Nitrite (in sample)
TON=Total oxidized nitrogen (NO3+NO2) in sample
A= NO3 concentration in mixed calibrant
B= NO2 concentration in mixed calibrant
Recovery = Coil efficiency (expressed as a decimal)
Phosphate (PO4)
Phosphate is analyzed using a modification of the Murphy and Riley (1962) technique. Similar to the silicate method, an acidic solution of ammonium molybdate is added to the sample to produce phosphomolybdic acid that is subsequently reduced to blue phosphomolybdous acid following the addition of ascorbic acid. Color intensity is directly related to the concentration of dissolved phosphate in the sample. The reaction product is then passed through a 10mm flow cell and the absorbance measured at 880nm.
Ammonia (NH3)
Ammonia is measured fluorometrically using a modification of the method described by Kerouel and Aminot (1997). In the presence of a borate buffer, samples are reacted with o-pthalaldehyde (OPA) to form a fluorescent complex that is excited at 370nm and emits at 460nm. The reaction takes place at 75°C. Sodium sulfite is added to the working reagent to reduce sensitivity to dissolved amino acids.
3. Method Notes
Ammonium is a difficult parameter to measure accurately due to its insidious nature and problems with contamination. Phosphorus and nitrogen compounds are also potential sources of contamination with poor sampling technique. Care must be taken during sampling to insure there is no contamination (e.g. touching the inside of the tube or the cap with fingers, smoking near rosette).
4. Water Sampling
Nutrient samples are drawn into 30 ml polypropylene screw-capped centrifuge tubes.
The tubes and caps are cleaned with 10% HCl and rinsed 3 times with sample before filling.
Samples that are not analyzed immediately are refrigerated and analyzed within 16 hours of collection. All samples are allowed sufficient time to reach room temperature. The centrifuge tubes fit directly onto the sampler.
5. Calculations
All data is reported in micro-moles/liter. The main calculations for concentration on the QuAAtro are run through the required AACE software interface. These calculations still follow the principle of other instruments, where:
[X] micro moles/liter =(Absorbance-blank) x F1 (Response Factor)
Values are corrected for drift based on changes in beginning and end standards, ultra pure water and the relative position of samples in the run. Corrections for linearity are performed, if necessary, based on a set of absorbances and concentrations; deviations from Beer’s law can be plotted to reveal a polynomial function that can be applied to correct sample values accordingly. Improvements in optics in the QuAAtro instrument have resulted in marked improvement in linearity and reduction of blank values for nitrate and silicate and phosphate.
6. Quality Control
A sample of reference material for nutrients in seawater (RMNS), produced by KANSO technos (www.kanso.co.jp) is included in every run and those data are monitored for consistency.
An aliquot from a large volume of stable deep seawater is run once a day as an additional check. The stability of the deep seawater check is aided by the addition of mercuric chloride as a poison.
The efficiency of the cadmium column used for nitrate reduction is monitored throughout the cruise and usually ranges from 97.0-100.0%.
NO3, PO4, NO2, and NH4 are reported to two decimals places and SiO2 to one.
Accuracy is based on the quality of the standards; the levels in micro moles/liter (µM ) are:
- NO3 = 0.05
- PO4 = 0.004
- SiO2 = 2-4
- NO2 = 0.05
-
NH3 = 0.03
The precision of the instrument for NO3, PO4, and NH4 is 0.01 μM and 1.0μM for silicate
and 0.01μM for NO2.
The detection limits in micro moles/liter for the instrumentation are:
- NO3+NO2 = 0.02
- PO4 = 0.02
- SiO2 = 0.5
- NO2 = 0.02
- NH3 = 0.04
7. Equipment/Supplies
Seal Analytical continuous-flow QuAAtro run by IOD since CalCOFI 1203SH; AutoAnalyzer 3 (AA3) run by ODF on cruises prior to 1203SH. Distributed by Bran and Luebbe, http://www.seal-analytical.com/
30 ml centrifuge tubes and test tube racks, 8 sets color coded and numbered
Barnstead Nanopure purified water system or equivalent polished water source
Sundry laboratory glassware
8. References
Armstrong, F.A.J., C.R. Stearns, and J.D.H Strickland, (1967). "The measurement of upwelling and subsequent biological processes by means of the Technicon Autoanalyzer and associated equipment," Deep-Sea Research, 14, pp.381-389.
Atlas, E.L., S.W Hager, L.I. Gordon, and P.K. Park, (1971). "A Practical Manual for Use of the Technicon AutoAnalyzer in Seawater Nutrient Analyses Revised," Technical Report 215, Reference 71-22, p.49, Oregon State University, Department of Oceanography.
Gordon, L.I., J.C. Jennings, A.A. Ross, J.M. Krest, (1992). "A suggested Protocol for Continuous Flow Automated Analysis of Seawater Nutrients in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study," Grp. Tech Rpt 92-1, OSU College of Oceanography Descr. Chem Oc.
Hager, S.W., E.L Atlas, L.I Gordon, A.W. Mantyla, and P.K. Park, (1972). " A comparison at sea of manual and autoanalyzer analyses of phosphate, nitrate, and silicate ," Limnology and Oceanography, 17, pp.931-937.
Keuroul, R. and A. Aminot, (1997). "Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis," Marine Chemistry Vol. 57, no. 3-4, pp.265-275.
Murphy, J. and J.P. Riley, (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimia Acta, Vol. 27 pp.31-36.
Subcategories
CalCOFI Handbook
The CalCOFI Handbook is a compilation of information for cruise participants. It explains many aspects of the science performed at sea, particularly the sample drawing methods for each sample type.
Data Formats
CalCOFI Data File Formats
Methods
CalCOFI standard practices for sample analysis, data processing, metadata & general methodology.
Software
SIO-CalCOFI software used at-sea and ashore, developed by the SIO CalCOFI Technical Group. Plus other software: auto-titrator oxygen analysis software developed by SIO's Ocean Data Facility; Seabird Seasoft & Data Processing software; Microsoft Office, Ultraedit, Ztree, hxD hex editor, Matlab, Surfer, Ocean Data View.

