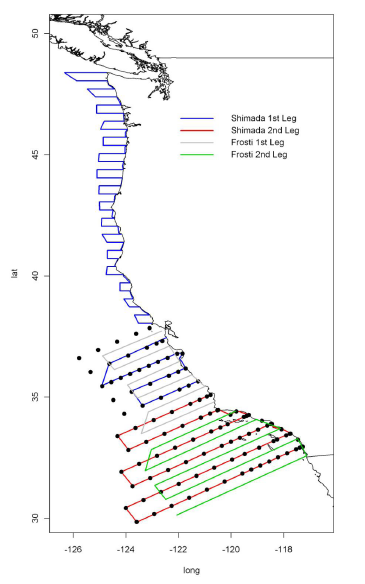
Itinerary
I. Cruise Overview
- Acoustic calibration 17 MAR – Seattle, Washington
- Leg 1: 17 MAR Dep. Seattle southwards, 30 MAR Arr. San Francisco
- Leg 2: 2 APR Dep. San Francisco, southwards, 7 APR Arr. waypoint Avila Beach
- Leg 3: 7 APR Dep. waypoint Avila Beach southwards, 27 APR Arr. San Diego. Offload.
I. Cruise Overview
A. Cruise Period: 17 March 2011 – 27 April 2011
B. Operating Area: Cape Flattery, WA to US/Mexican border with variable transect lengths (please refer to Appendix 1 for detailed plot).
C. Summary of Objectives: Survey the distributions and abundances of pelagic fish stocks, their prey, and their biotic and abiotic environments in the area of the California Current between Cape Flattery, Washington and San Diego, California. The following are specific objectives for legs I & II (Daily Egg Production Method, DEPM) and leg III (Spring CalCOFI).
Legs I & II (DEPM):
Continuously sample pelagic fish eggs using the Continuous Underway Fish Egg Sampler (CUFES). The data of Pacific sardine eggs will be used to allocate additional Pairovet samples to estimate the daily egg production of Pacific sardine. The Pairovet samples will be also taken at predetermined stations. Both samples from CUFES and Pairovet will be used to estimate the distributions and abundances of spawning sardine, anchovy and mackerel and other species.
2. Continuously sample multi-frequency acoustic backscatter using the Simrad EK60. The data will be used to estimate the distributions and abundances of coastal pelagic fishes (e.g., sardine, anchovy, and mackerel) and krill species.
3. Sample selected aggregations of fish and zooplankton which have been observed acoustically. These data will be used to identify the sound scattering species and their sizes.I.C.4 Sample fish near the surface at nighttime by conducting 2-5 surface trawls at stations (Appendix 2) or at random sites each night. The data will be used to estimate the reproductive parameters, distributions and demographics of sardine, anchovy and mackerel.
5. Continuously sample profiles of currents using the RDI/Teledyne Acoustic Doppler Current Profiler.
6. Continuously sample sea-surface temperature, salinity, and chlorophyll-a using a thermosalinograph and fluorometer. These data will be used to estimate the physical oceanographic habitats for target species.
6. Continuously sample sea-surface temperature, salinity, and chlorophyll-a using a thermosalinograph and fluorometer. These data will be used to estimate the physical oceanographic habitats for target species.
7. Continuously sample air temperature, barometric pressure, and wind speed and direction using an integrated weather station.
8. Sample profiles of seawater temperature and salinity using a CTD with water-sampling rosette and other instruments at prescribed stations.
9. Sample plankton using a CalBOBL (CalCOFI Bongo) at prescribed stations. These data will be used to estimate the distributions and abundances of ichthyoplankton and zooplankton species.
8. Sample profiles of seawater temperature and salinity using a CTD with water-sampling rosette and other instruments at prescribed stations.
9. Sample plankton using a CalBOBL (CalCOFI Bongo) at prescribed stations. These data will be used to estimate the distributions and abundances of ichthyoplankton and zooplankton species.
10. Sample plankton using a Manta (neuston) net at prescribed stations. These data will be used to estimate the distributions and abundances of ichthyoplankton species.
11. Sample the vertically integrated abundance of fish eggs using a Pairovet net at prescribed stations. These data will be used to quantify the abundances and distributions of fish eggs.
Leg III (Spring CalCOFI):
12. Continuously sample pelagic fish eggs using the Continuous Underway Fish Egg Sampler (CUFES). The data will be used to estimate the distributions and abundances of spawning sardine, anchovy and mackerel.
13. Continuously sample multi-frequency acoustic backscatter using the Simrad EK60. The data will be used to estimate the distributions and abundances of coastal pelagic fishes (e.g., sardine, anchovy, and mackerel), and krill species.
14. Continuously sample sea-surface temperature, salinity, and chlorophyll-a using a thermosalinograph and fluorometer. These data will be used to estimate the physical oceanographic habitats for target species.
15. Continuously sample air temperature, barometric pressure, and wind speed and direction using an integrated weather station.
16. Sample profiles of seawater temperature, salinity, chlorophyll-a, nutrients, and phytoplankton using a CTD with water-sampling rosette and other instruments at prescribed stations. Measurements of extracted chlorophyll and phaeophytin will be obtained with a fluorometer. Primary production will be measured as C-14 uptake in a six hour in situ incubation. Nutrients will be measured with an auto-analyzer. These data will be used to estimate primary productivity and the biotic and abiotic habitats for target species.
17. Sample the light intensity in the photic zone once per day in conjunction with a daytime CTD station. These data will be used to interpret the measurements of primary production.
18. Sample plankton using a CalBOBL (CalCOFI Bongo) at 10 nmspaced stations. These data will be used to estimate the distributions and abundances of ichthyoplankton and zooplankton species.
19. Sample plankton using a Manta (neuston) net at prescribed stations. These data will be used to estimate the distributions and abundances of ichthyoplankton species.
20. Sample the vertically integrated abundance of fish eggs using a Pairovet net at prescribed stations. These data will be used to quantify the abundances and distributions of fish eggs.
21. Sample plankton using a PRPOOS (Planktonic Rate Processes in Oligotrophic Ocean Systems net) at all prescribed CalCOFI stations on lines 90.0 and 80.0 as well as stations out to and including station 70.0 on lines 86.7 and 83.3. These data will be used in analyses by the LTER (Long Term Ecological Research) project.
22. Continuously sample profiles of currents using the RDI/Teledyne Acoustic Doppler Current Profiler.
23. Continuously observe, during daylight hours, seabirds and mammals. These data will be used to estimate the distributions and abundances of seabirds and marine mammals.
24. Addition to usual CalCOFI protocol: Sample fish near the surface at nighttime by conducting 1 trawl between 30 - 60 minutes after the start of each night (defined as 30 minutes after sunset). Add one trawl prior to each of the next two CalCOFI nighttime stations, before deployment of the pairovet/ bongo/ manta net tows. Transit between nighttime stations at 14 knots. The trawl data will be used to obtain species composition and size structure for partitioning acoustic backscatter attributed to fish. No trawling at CalCOFI stations will be done during daylight. No changes to the location of the CalCOFI stations will be made. After the CalCOFI grid is complete, all extra days will be used to fill gaps in the daytime acoustic transects and do more nighttime trawling in areas of high Coastal Pelagic Species abundance, and to conduct some directed trawls during daytime.
Participating Institutions
- Southwest Fisheries Science Center, Fisheries Resources Division.
- Scripps Institution of Oceanography, Integrative Oceanography Division.
- Monterey Bay Aquarium Research Institute.
Points of Contacts:
- Chief Scientist/alternate: Sam McClatchie/David Griffith (858-546-7083/858-546-7155; 8604 La Jolla Shores Drive, La Jolla, CA, 92037; This email address is being protected from spambots. You need JavaScript enabled to view it./This email address is being protected from spambots. You need JavaScript enabled to view it.),
- Ops Officer: LT Amanda Goeller (This email address is being protected from spambots. You need JavaScript enabled to view it.)
- Ship cell phone (206) 427-2374
- Ship Iridium phone (808) 684-5457 code: 8816 5145 2194