 Frequently Asked Questions About Fluorometric Chlorophyll Analysis
Frequently Asked Questions About Fluorometric Chlorophyll Analysis
Q: Why measure chlorophyll?
A: All plant life contains the primary photosynthetic pigment chlorophyll a. Microscopic, planktonic plants, or phytoplankton, occupy the lit zone of all water bodies. With over 70% of the surface of the earth covered in water, phytoplankton and photosynthetic bacteria are responsible for almost ½ of the planets primary production while their total biomass comprises less then 1% of the total plant biomass. These extraordinarily efficient plants also act as the single largest CO2 sink on earth. For these reasons alone it should be clear that there is an interest in measuring concentrations of phytoplankton. Chlorophyll a fluorescence is the most versatile, sensitive and easy way to measure the concentrations of phytoplankton in water.
The quantitation, through extracted analysis, or estimation, through in vivo analysis, of chlorophyll a concentration supplies information on the abundance of phytoplankton present in all aquatic environments. Since chlorophyll-containing organisms are the first step in most food chains, the health and /or abundance of these primary producers will have cascading effects to all higher organisms. Therefore, the determination of chlorophyll concentration is one of the key indices in monitoring the health of any natural system.
Chlorophyll measurements are also used to directly monitor phytoplankton populations. Examples include, but are not limited to, the monitoring of chlorophyll in natural marine and freshwater environments, reservoirs, water and sewage treatment plants, and aquacultural systems.
Q: How do fluorometers detect and quantify chlorophyll a in water?
A: Fluorescence is the phenomena of some compounds to absorb specific wavelengths of light and almost instantaneously emit longer wavelengths of light. Chlorophyll a naturally absorbs blue light and emits, or fluoresces, red light. Fluorometers detect chlorophyll a by transmitting an excitation beam of light in the blue range (440nm for extracted analysis and 460nm for in vivo analysis) and by detecting the light fluoresced by cells or chlorophyll in a sample at 685nm (red). Generally, this fluorescence is directly proportional to the concentration of the material in question.
Q: What is the difference between in vivo, in vitro , and extracted chlorophyll analysis?
A: In vitro (meaning 'in glass' and referring to 'in an artificial environment or outside the living organism') chlorophyll analysis is another term for extracted analysis. It entails the concentration of chlorophyll containing cells onto a filter followed by the extraction of the chlorophyll a from the cells. In vivo (meaning 'within a living organism') chlorophyll analysis simply refers to the analysis of chlorophyll in the natural environment or, in our case, in the living algal cells.
Q: What is in vivo chlorophyll analysis?
A: In vivo chlorophyll analysis is the fluorescent detection of chlorophyll a in living algal and cyanobacterial cells in water. In this technique, the excitation light from the fluorometer passes through the untreated sample water and excites chlorophyll within the living cells of the algae present. There are several factors that make in vivo analysis a semi-quantitative measure at best. Environmental parameters, physiology, morphology, light history and the presence of interfering compounds all play a role in altering the relationship between fluorescence and the concentrations of chlorophyll a. Examples of interfering materials include other plant pigments, degradation products, dissolved organic matter, and turbidity. In vivo fluorescence data supplies information on the relative distribution of chlorophyll concentrations and usually correlate well with extracted chlorophyll a samples.
In vivo detection has several very useful applications. An example is the monitoring of general trends in chlorophyll concentrations in real time. It is very easy to obtain large amounts of data using in vivo instrumentation and is an excellent means of following trends and estimating chlorophyll concentration. With the introduction of the Wetlabs® submersible fluorometer, verticals profiling and mooring applications are now possible. Other examples of in vivo applications include continuous monitoring along a ship's track using the 10-AU configured with a flowcell and discrete sampling used to monitor algal concentrations in natural or laboratory phytoplankton populations. Examples of discrete sampling applications include aquaculture and hatchery systems, water treatment facilities, reservoir monitoring, and aquatic research (see the Aquafluor™).
If water samples are taken, the in vivo data can be correlated to extracted chlorophyll a data to estimate actual concentrations. Otherwise, the in vivo data can be used as a relative measurement to identify trends and patterns.
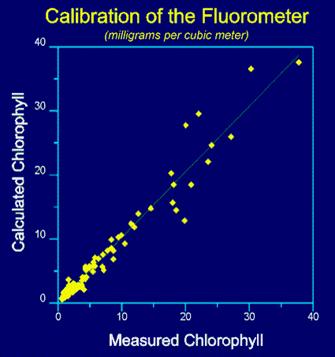 Q: How do you calibrate a fluorometer for extracted chlorophyll-a analysis?
Q: How do you calibrate a fluorometer for extracted chlorophyll-a analysis?
A: Allow the fluorometer to warm-up for the time specified in the User's Manual. Measure the fluorescence of each standard at sensitivity settings that provide mid-scale readings (refer to your User's Manual for proper calibration procedures). Follow directions under section 10.0 from E.P.A. Method 445.0 (Revision 1.2) for the calibration and standardization procedure using the traditional acidification technique or the non-acidification method. E.P.A. Method 445.0 calls for filtering onto glass fiber filters (GFF) filters and grinding of the filters. This step may not be necessary in some systems and tests should be run to compare extractions with and without grinding. Non-grinding techniques can use either GFF or membrane filters that will dissolve in the solvent.
Q: What environmental factors interfere with in vivo chlorophyll analysis?
A: Light, temperature, water quality, and dissolved components can all have significant effects on fluorescent readings independent of the chlorophyll concentration. However, all of these factors can be controlled and/or corrected to a degree if the user is aware of their effects.
Temperature has an inverse relationship with fluorescence. For example, in a vertical profile, as the temperature decreases, the fluorescence will increase independent of chlorophyll concentration. The in vivo chlorophyll fluorescence response changes at a rate of 1.4% per °C. A temperature drop of 10 °C in a vertical profile would result in a 14% overestimation of chlorophyll at the coldest point. Turner Designs' field and submersible instruments have the capability to automatically compensate for temperature effects.
Light history can have significant affects on the fluorescence in algal cells. For example, at low light levels, algal cells can optimize the light uptake by pushing chloroplasts to the outer edge of the cell or by producing more chlorophyll per cell. Both of these responses can result in data increases the fluorescence signal while the algal biomass may be unchanged. To lessen the effects, opaque hose should always be used when sampling natural waters with a field fluorometer. The transport time of the water in the hose will dark-adapt cells to an extent, significantly reducing fluorescence error caused by variations in the light history of the cells.
Dissolved organic matter (DOM), chlorophyll degradation products (pheophytins), chlorophyll b & c and turbidity can also falsely increase the chlorophyll a fluorescence signal. If the dissolved interfering compounds are suspected to be significant, it is worth conducting a quick study to look at the effects by comparing the fluorescence from filtered and non-filtered water samples or from noting the fluorescence signal below the photic zone and using this value as a blank.
Q: What is the effect of varying species on fluorometric chlorophyll analysis?
A: Different species of phytoplankton have varied morphologies (cell packaging), physiological states, size, and chlorophyll a : carbon ratios. All of these factors can affect the fluorescence emitted from a cell under a specific excitation light intensity. Luckily, in natural environments, the phytoplankton assemblage is diverse enough that much of the variation in fluorescence resulting from the factors above balance eachother out so the net effect on the fluorescence reading is reduced. Nevertheless, the researcher needs to be aware of the potential for error in readings caused by these factors to better interpret in vivo data.
 Q: How does other chlorophylls and degradation products affect extracted chlorophyll analysis?
Q: How does other chlorophylls and degradation products affect extracted chlorophyll analysis?
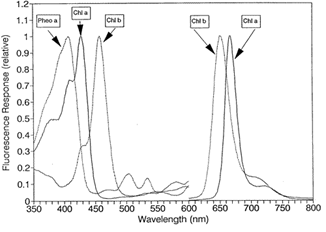
A: All chlorophyll pigments and their degradation products (pheophytins) have their own unique excitation and emission spectra. Unfortunately, these spectra overlap significantly with the fluorescence spectra of chlorophyll a due to the similar chemical structure (see Figure 2). Due to the overlap in fluorescence spectra, the presence of one can result in an interference in the measurement of another, resulting in an under or overestimation of the pigment in question.
In the case of chlorophyll a, interfering pigments to be aware of are chlorophyll b, chlorophyll c, and pheophytina(see figure 3). Chlorophyll b causes the most interference in freshwater systems with high concentrations of chlorophytes and/or prasinophytes and in marine systems with high concentrations of prochlorophytes. In chlorophyll extraction, the interference results during the acidification step of the traditional extraction technique. Chlorophyll b undergoes a wavelength shift when acidified, resulting in an underestimation of chlorophylla and an overestimation of pheophytin. In environments with high chlorophyll b concentrations, we strongly recommend using the Welschmeyer (non-acidification) method.
High concentrations of chlorophyll c can result in a slight overestimation of chlorophyll a and an underestimation of pheophytin a sometimes even resulting in negative pheophytin readings. It has been reported that a chla :chlb ratio of 1 : 1 , which is the highest ratio which could occur in nature, would result in achla overestimation of 10%.
High concentrations of any of the interfering compounds will result in an increase to in vivo chlorophyll readings. The magnitude of the increase will depend on the instrumentation used. Instruments using filters with a wider bandpass will be more effected by interfering compounds than instrument with narrower bandpass filters.
Q: What is a secondary standard?
A: A secondary standard is used as an alternative to a primary calibration standard. It is often used when primary standards are expensive, difficult to obtain, or unstable. In the case of chlorophyll a, a secondary standard can be used the majority of time for calibration because liquid chlorophyll a standards are expensive, time-consuming, and photosensitive. To properly use a secondary standard, you must first calibrate with a primary liquid standard of the fluorophore of interest. You can then obtain the equivalent value of the secondary standard. Henceforth, you may calibrate using the secondary standard using the value you obtained for it initially. An occasional calibration using a primary standard to recheck the stability is recommended.
In the past, secondary standards have been more stable than the primary standards they mimic, but have still required special storage and handling conditions with relatively short lifetimes. Examples of these include coproporphrin and fluorescent dyes such as Rhodamine WT.
Turner Designs has developed a solid secondary standard that is stable under ambient light and temperatures with no special treatment or storage required. This new secondary standard will greatly reduce time, cost, and trouble in fluorescent chlorophyll analysis procedure.
Q: What is the best solvent and procedure for the extraction of chlorophyll a?
A: The most commonly used extraction solvent is a 90% acetone 10% DI water solution. Other solvents, such as methanol, ethanol and acetone/DMSO mixtures are also commonly used and can improve extraction efficiency with specific phytoplankton or may be found useful for the extraction of sediment samples.
There is no 'best' solvent or procedure for chlorophyll extraction. Several work well and have their own pros and cons. The E.P.A. Method 445.0 describes the recommended step-by-step process for analysis using 90% acetone. There are many factors in the extraction process that can lead to different results. Several examples of these factors will be discussed below in hopes reducing some of the variability.
An excellent resource for sample collection, storage and extraction methods is the UNESCO publication, Phytoplankton Pigments in Oceanography.
Water collection, storage, and filtration:
Because pigment is being extracted from living cells, it is critical to use consistent techniques of obtaining water, filtering and storing filters. The living cell is sensitive to changes in the environment such as temperature and light. Conditions leading to cell death or damage will affect chlorophyll concentrations.
In the collection of water samples, it is important to make certain that the collection containers are clean of all chemicals. They should be rinsed several times in the sample water. Once collected, if samples cannot be filtered immediately, they should be stored quickly on ice in the dark. The time between collection and filtration should be as brief as possible and should not exceed 4 hrs.
Specifics on the recommended filtration and storage procedure can be found in E.P.A. Method 445.0. An excellent resource for sample collection, storage and extraction methods is the UNESCO publication, Phytoplankton Pigments in Oceanography.
Q: How do I take and store discrete water samples in the field?
A: For discrete in vivo analysis, water samples should be measured as soon as possible after collection. The same time constraints should be placed on the filtering of water samples that are to be used for extracted analysis. From the time of collection to measurement, the samples should be stored in the dark on ice. Remember that the cells are living and significant time in a container will alter the physiological state of the algal cells, resulting in misrepresented chlorophyll data compared to the natural situation.
Discrete samples need to be kept at the same temperature. This is most easily accomplished through the use of a water bath. The bath should be covered from direct light. When the samples are being measured in the fluorometer, a 'time in the instrument' must be established. Use the discrete sample averaging function on the 10-AU or TD-700 Fluorometer or wait for the reading to stabilize (~10 seconds) and record the fluorescence. If this time is not monitored the heat and light in the instrument will cause fluorescence to change.
When developing you own sample collection and storage procedures, it is recommended to run your own experiment to test change in fluorescence over a given transport time by analyzing samples from a given sample at hour or half-hour intervals.
Q: How do I calculate actual chlorophyll a concentrations from my fluorometric data?
A: ACIDIFICATION METHOD
Prior to running sample on the fluorometer, the instrument must be calibrated with a pure chlorophyll a standard and the maximum acid ratio must be determined by measuring the fluorescence of the standard before and after acidification. If afluorometer other than a digital Turner Designs instrument is being used, the fluorometer sensitivity coefficient may also need to be determined. For further information please refer to EPA Method 445.0.
chl a =K (Fm/ Fm -1) x (Fb-Fa) x (v/V)
pheo a =K (Fm/ Fm -1) x [(Fm x Fa - Fb)] x (v/V)
If necessary, the result can be multiplied by a dilution factor.
where:
K= sensitivity coefficient, equal to 1 on 10-AU
Fm = max acid ratio Fb/Fa of pure chlorophyll a standard
Fb = fluorescence before acidification
Fa = fluorescence after acidification
Fo = fluorescence signal of sample
v = extract volume (L)
V= volume filtered (L)
NON-ACIDIFICATION METHOD (Chlorophylla concentration only)
1) Collect fluorescence data (one number/sample)
** DO NOT ACIDIFY**
2) Plug data into following equation:
chl a = (Fo x v)/ V
where:
Fo = fluorescence signal of sample
v = extract volume (L)
V= volume filtered (L)
Q: Does the E.P.A. approve fluorometric chlorophyll analysis?
A: Yes, the E.P.A. has published Method 445.0 which covers the in vitro(extraction) fluorometric analysis of chlorophyll a. In the most recent revision (Rev 1.2 Sept., 1997), the E.P.A. also approves the use of the non -acidification method, which is less susceptible to interfering compounds such as chlorophyll b. This filter kit supplies only chlorophyll a concentrations with no information on pheophytin concentration.
Q: How can I compare chlorophyll data obtained through different measurement techniques?
A:All detection instrumentation used in chlorophyll analysis will result in chlorophyll concentrations that are directly comparable. A side-by-side comparison between a fluorometer and a spectrophotometer is easily done but would require dilution of the chlorophyll sample to put it within the linear range of the fluorometer. A sample that is in range on a spectrophotometer will be over-range on a fluorometer.
Q: Why use a fluorometer over a spectrophotometer for extracted chlorophyll analysis?
A: Benefits of fluorescence over spectrophotometry include the capability of in vivo detection, sensitivity, durability, versatility (accepts a wide range of discrete sample cells and flow cells, accepts AC or DC power, and the user can choose and quickly change between many optical kits), ease of use, stability, ease of transport, and a small footprint.
For oceanographic research, the greater sensitivity of fluorescence results in less time and work in the analysis because much less water must be filtered for extracted analysis. The superior sensitivity also enables in vivo detection of chlorophyll concentration of <1µg/L.
Freshwater researchers now have an extremely accurate and easy way to measure chlorophyll a even with high chlorophyll b concentrations using the non-acidification optical kit. Fluorometers also allow for in-line monitoring to collect data in real time.
Q: What are the chlorophyll detection limits of Turner Designs Fluorometers?
A: The TD-700 Laboratory Fluorometer and the 10-AU Field Fluorometer with a red sensitive photomultiplier tube (PMT), have extracted chlorophyll detection limits of 0.02µg/L using a 13mm diameter test tube and 0.01µg/L using a 25mm test tube. The SCUFA® Submersible Fluorometer can detect in vivo chlorophyll concentrations to 0.02µg/L.
